A recent Guardian article from Birmingham University biostatistics Professor John Deeks –https://bit.ly/34X9e9r – warned against cutting scientific corners in the pursuit of the ultimate COVID-19 antibody test.
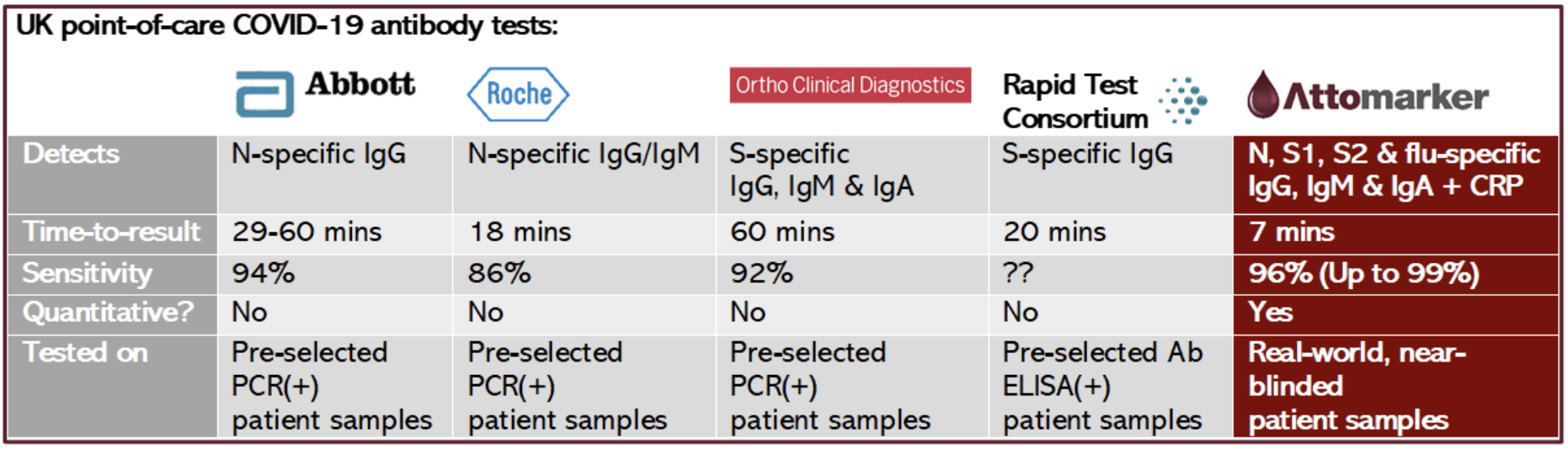
It had become apparent that the ‘98% sensitivity’ reported for the UK Rapid Test Consortium test was gained through testing on patients pre-screened with an inaccurate, lab-based (ELISA) antibody test. This means that only patients with serious disease (and therefore high levels of antibodies) were likely to be tested.
Previously in May, a new test from Roche had been widely reported as ‘100%’ accurate but Public Health England showed that the test failed to identify 16% of samples from people who had COVID-19. And in June, public sales of many laboratory standard antibody tests were stopped after the Medical & Healthcare products Regulatory Agency found they were using finger-prick samples rather than the larger venous blood samples required.
The Rapid Test Consortium had been favoured due to a perceived need to promote British technology in the battle against the pandemic after the UK Government had earlier purchased ineffective antibody tests from China.
The fact is, the best antibody test has already been manufactured by a British spin-out from the University of Exeter and has been MHRA-approved since July.
Attomarker’s multiplex test is the most rapid (7 minutes), the most sensitive and the only quantitative point-of-care COVID-19 antibody test available in the UK. It is the only one to have been trialled on a real-world, near-blinded 200-patient cohort in St Thomas’ Hospital, London, peer-reviewed in the journal of the Royal Society of Chemistry – https://rsc.li/2F2lIS2 – with a further accuracy analysis now close to publication.
Attomarker is in close communication with the Department of Health & Social Care & Innovate UK, and is currently in advanced conversations with potential investors, seeking £5m for the nationwide rollout of its ultimate COVID-19 antibody testing solution.